Before a specific target protein can be singled out and investigated in detail, a large number of proteins have to be evaluated in high-throughput screenings (HTS). To focus on their function without the influences of the other molecules present in the organism, these proteins have to be purified. Many high throughput purification applications require expensive equipment and specific technical knowledge. Alternatively, well-known and already established methods can be scaled up to be carried out in any size of lab.
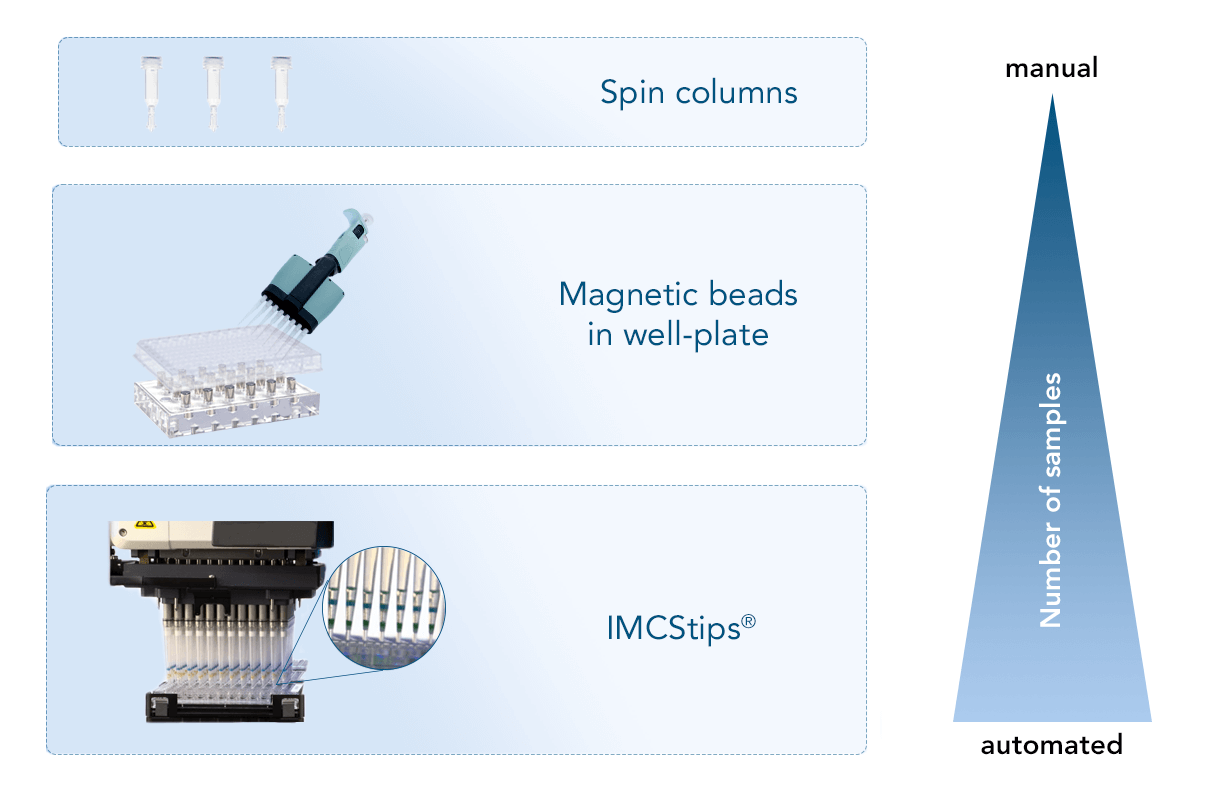
Manual applications
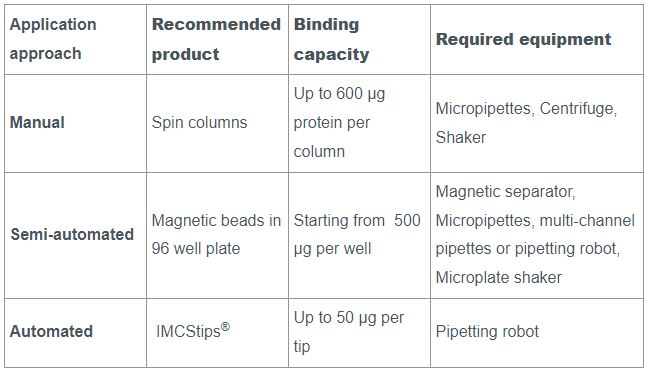
For small-scale experiments with a limited number of samples, spin columns can be used. They are a convenient tool for small sample volumes that can still be handled manually. Thereby the number of proteins that can be purified at the same time in a fast and efficient way is flexible. A Spin Column Kit with all necessary components for protein purification, as well as Empty Spin Columns that can be combined with the customer’s resin of choice are available from IBA Lifesciences.
Semi-automated applications
Magnetic beads, such as MagStrep “type3” XT beads, can be used in a round-bottom 96-well plate, allowing the simultaneous purification of up to 96 samples. In this format, the application can still be carried out manually, but the use of pipetting robots is also possible. The magnetic core of the beads allows the use of a magnet to fix the beads to the wells when removing the supernatant, diminishing the loss of beads and eliminating the need for centrifugation.
Automated applications
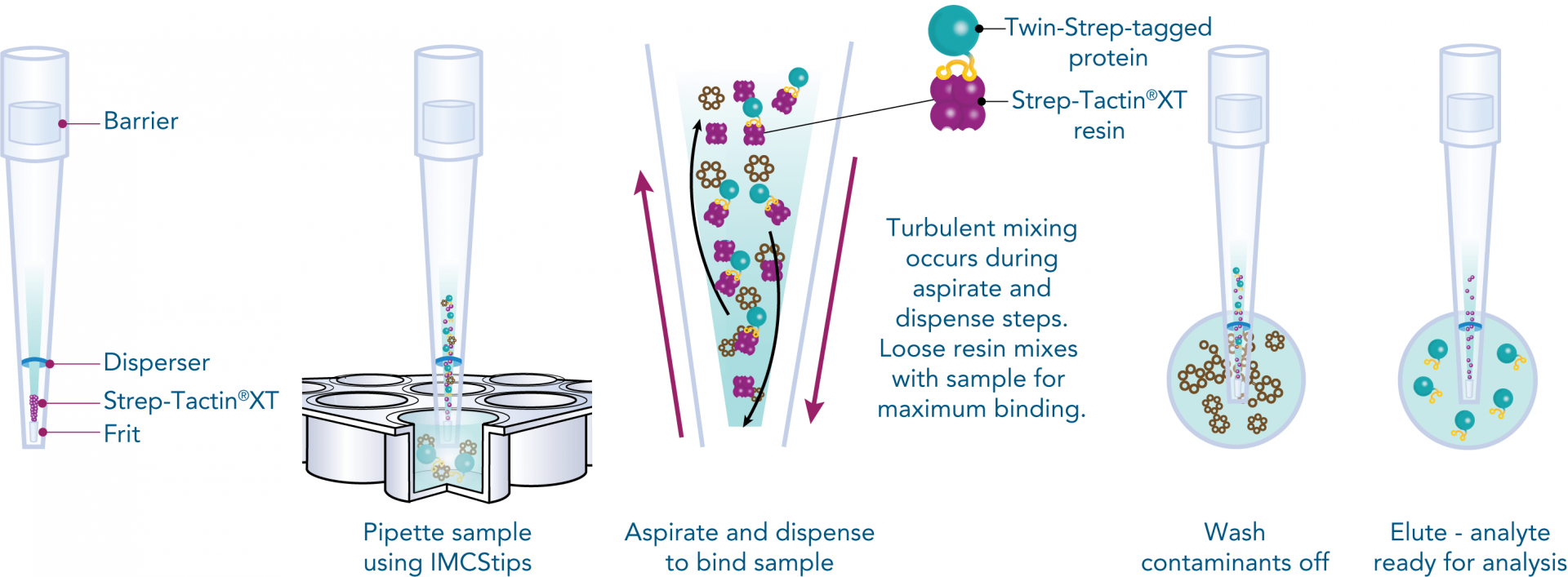
For an automated approach that requires minimal manual intervention, IMCStips® containing Strep-Tactin®XT resin are available. The tip format allows high-throughput affinity purification with consistency and reproducibility making it especially attractive to industrial users. The approach is described in detail in the following application note. You can learn more about the automated purification of proteins using IMCStips® on the IMCS website.
Analytical High-throughput Screening Applications
The identified and purified proteins from the initial screening can then be further characterized by using the Strep-tag® technology. Due to the outstanding high affinity of the Twin-Strep-tag® to Strep-Tactin®XT (pM Range), Strep-Tactin®XT is very well suited for biomolecular interaction analysis technologies as Surface Plasmon Resonance (SPR) or Biolayer interferometry (BLI).